
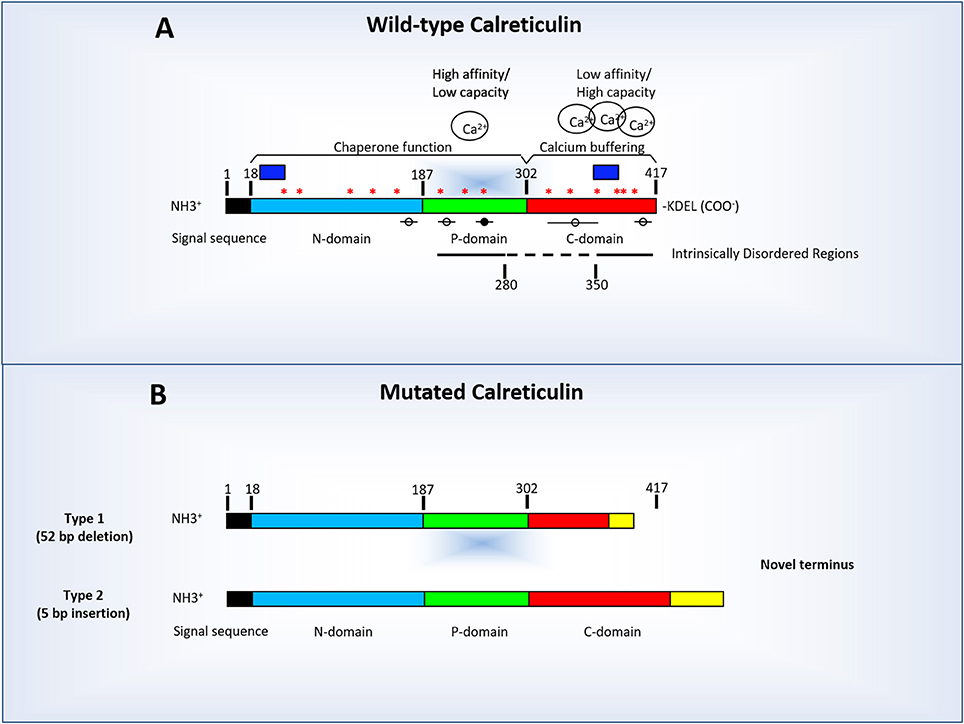
Mutagenesis of two PKC/PDK residues in CNX indicated that S562 supports phosphorylation. Unlike CRT, which is entirely lumenal, CNX has a cytosolic domain that is phosphorylated by multiple kinases. Coexpression of SERCA2a (which lacks the luminal asparagine) with either CRT or CNX does not inhibit Ca 2+ oscillations, consistent with the notion that a lectin interaction may be involved. The mechanism of inhibition may involve a lectin-like interaction since mutagenesis of a lumenal asparagine to alanine in SERCA2b destroys the inhibitory effect. By domain deletion mutagenesis of CRT we have determined that the N and P domains are necessary for the inhibition of Ca 2+ oscillations. CRT and CNX overexpression inhibit Ca 2+ oscillations when co-expressed with SERCA2b or when oocytes are treated with pyruvate malate to induce oscillations. Following release, Ca 2+ is re-sequestered into the ER by Ca 2+ ATPases of the SERCA family. In Xenopus oocytes and other cells, stimulation by G-protein and tyrosine coupled receptors results in Ca 2+ release from the Inositol 1,4,5 trisphosphate receptor (IP 3R) located in the ER. Aside from their role as foldases in the ER, our laboratory has shown that all members of this family of proteins modulate Ca 2+ oscillations. Calreticulin (CRT) and calnexin (CNX) are members of a family of endoplasmic reticulum (ER) chaperones that fold newly synthesized polypeptides.


 0 kommentar(er)
0 kommentar(er)
